
2025 Oʻahu
Weed Control and Restoration Workshop
This workshop was brought to you by the Priority ONE (Oʻahu Native Ecosystems) Working Group. Members of the organizing team represented the Waiʻanae Mountains Watershed Partnership, DOFAW's Native Ecosystem Protection and Management, Kualoa Ranch, the Army Natural Resources Program, Oʻahu, and the Ecosystems Extension Program. The agenda was also developed with input from the Koʻolau Mountains Watershed Partnership. Mahalo to those of you who completed our pre-workshop surveys, your input contributed to development of the workshop goals and content. Mahalo nunui to PCSU for sponsoring the venue.
The workshop featured a set of presentations, restoration topic breakout group discussions, and an activity geared towards increasing Herbicide labels fluency and setting up Herbicide trials. Below is a brief overview of these sessions. Navigate to a specific section with the side menu.
Invited presenters gave updates about LFA on Oʻahu (OISC), coqui frog on Oʻahu (DOFAW and partners), biocontrol development for Natural Area weeds (USDA-FS), and an overview of the inaugural meeting of the Hawaiʻi Drone Hui. Watch the presentations, review the presentation slides and see what questions the audience asked.
The breakout group discussions centered on three restoration topics 1) CRB in restoration sites, 2) Nursery germination--successful methods, challenging species and trials for about 24 species, and 3) seed-based restoration trials. See the posters that were prepared, synthesis of the CRB and Nursery Germination discussions and supplemental information about ANRPO direct seeding trials.
The herbicide trial activity was based around Cinamommum bermanii, Trema orientalis, Spathodea campanulata, Syzygium cuminnii, Ficus spp., Citharexylum spp., Rubus argutus, Cenchrus ciliaris, and Megathyrsus maximus. DOFAW-NEPM presented an example of establishing herbicide trials to determine a control method for Stapelia gigantea. Breakout groups focused on one of the plant species listed above. Each group walked through key steps for setting up an herbicide trial for that species, following a worksheet structured similarly to the process presented by DOFAW-NEPM. Read how the species above were selected, view the presentation and download the worksheet for your own herbicide trial design.
Presentations
Click on the thumbnail to watch the presentation (will redirect to YouTube, except for the LFA presentation). Click on the PDF icon to view the presentation slides. Read more to see the questions from the audience.
Do you have additional questions? Feel free to reach out to EcosystemsExtensionHI@gmail.com to continue the conversation!

LFA Update
Presenter: Erin Bishop (OISC)
Overview of Little Fire Ant (LFA) status across state and on Oʻahu, LFA biology, human-LFA interactions that facilitate invasion, control strategies (monitoring, detection, treatments, etc.)
Presentation
Slides
Q: What is the timeframe for eradication? A: Treatments every 6 weeks, for 12 months
Q: How do you treat in trees? A: Toxicant (granular) on the ground, spray growth inhibitor (liquid) in trees
Q: If your greenhouse is LFA free, how often do you recommend testing? Monthly? Is quarterly sufficient? A: Testing quarterly is sufficient, if you are LFA free you can even test annually.
Q: In nursery, what is spacing of sample sticks/vials? A: Every 10 steps
Q: Is there an LFA certification for nurseries? A: No
Q: Is there language for contracts when buying plants from nurseries? A: Yes, can reach out to OISC for language/SOPs that will ensure folks test for LFA. Or can have OISC check your language
Q: What is the main vector? A: Plants, landscaping. Large landscaping projects can spread ants very quickly. Not all nurseries have LFA but those with detections are working with HDOA. Just because a nursery didnʻt find LFA there in the past does NOT mean that they are LFA free, need to test consistently to be confident of this. Quarantine and test plants from nurseries.
Coqui Frog Update
Presenters: Ryan Chang (DOFAW), Jessica Miura (HDOA), Robbie Snyder (OISC), Kimeona Kane (Waimānalo Lineal Descendent, Waimānalo Neighborhood Board), Taylor Campbell (Waimānalo Agriculture Association), Timo Sullivan (Aloha ʻĀina Drones)
Presentation
Slides
Overview of Coqui frog invasion on Oʻahu, History of coqui frog control efforts, Waimanālo community initiative, collaboration between OISC, DOFAW, HDOA, and communities impacted by coqui frog, application of innovative drone methodologies for survey and control of coqui frog with Aloha ʻĀina Drones
Q: How do you catch coqui? A: With a long tube. Listen for frog, position tube opening in front of frog and frog will jump in. Getting harder to find tubes as flouro lights get phased out. In need of plastic bottles to store caught frogs, give us your Gatorade bottles!
Biocontrol Update
Presenter: Tracy Johnson (USDA-FS)
Status of biocontrol agents for:
An assortment of Melastomes [e.g., Chaetogastra (previously known at Tibouchina), Miconia calvescens (Miconia) and Miconia crenata (Clidemia)]
Falcataria molucanna (Albizia)
Hedychium gardnerianum (Himalayan ginger)
Rubus spp
Schinus terebinthifolius (Christmas berry)
Chromolaena odorata (Devilweed)
Hawaiʻi Drone Hui Overview
Presenter: Dan Jenkins (UH Mānoa, CTAHR)
Overview of:
1) background (history and trends) in aerial application of pesticides
2) existing (and upcoming?) regulatory framework
3) innovative applications for conservation/NRM in Hawaiʻi
Presentation
Slides
Q: What are the challenges to using drones that were discussed at Drone Hui meeting? A: regulations that limit flights to line of site; piloted aviation?; operating at night (automated flights have proven to be a good workaround for this, if can fly in daytime, can fly in nighttime); certain targets are easier than others (aka ones that are low to the ground are harder?); variety in terrain, elevations of use; have to prep sites
Poster Gallery:
Restoration topics
Mahalo to all the programs and individuals who contributed posters to this session. Peruse the posters, click to enlarge, or use side menu to navigate to Summary Tables extracted from the posters below.
















CRB in Restoration
The table below synthesizes the CRB information shared across programs. Use the drop down menu to filter the table by Management Actions specific to Greenwaste, Dead Palms or Live Palms. Link to to posters which the information was drawn for more information about the program, their sites and CRB management. At this time, you will also find a link to the Priority ONE "CRB in Restoration" discussion synthesis.
Note that the programs represent a mix of environments, spanning developed and natural areas. The programs manage a mix of native ecosystem restoration sites, native palm populations, or non-native palm collections. None of these programs are situated within dense urban areas and are often dealing with specific sets of challenges due to access and topography.
CRB Discussion
Will there be testing for control methods on species other than Palms? Not too many other preferred species (banana, hala, etc.) available at the gardens, but might want to explore control methods on kalo next. There are a lot of collections in the gardens.
What herbicides are you using/testing? Merit is a granular herbicide, but probably not a long-term solution. Drenching takes too long and is not feasible. Crown injection methods also consume staff time. There is money to fund a full-time team of two for four months all performing crown injections on coconut.
Have there been any trees that came back after it was attacked? No rares have come back, but there was one from Hoʻomaluhia that regrew after being eaten by CRB.
Is it worthwhile to remove a standing dead tree? These are often the lowest on the totem pole for removal at the gardens and could take 4-5 months to get to it. Maybe if people/landowners can do it themselves by netting the trunk (goal is to get the slowest decay). Cutting and subsequent chipping might get everything already in the pile, but would not control the eggs. Aerial treatment of imidacloprid could work, no granular or vascular treatments would work.
Does a thick canopy “hide” palms species beneath? CRB tend to fly high and mostly hit taller trees. At first, the decision was to halt planting more loulu at the gardens, but will reconsider the decision. Decoy trees might be a good distraction.
Are there ways to systematically search the soil? No, we usually find them while outplanting and through moving the soil around, we find grubs in the soil.
Yellow Crazy Ants possibly preying on the CRB, though unclear if they are deliberately going after them or finding them as food after the fact.
Does the sun/UV deteriorate the plastic netting? Yes, deteriorating netting will create large piles of nets with holes and eventually microplastics. There are two types of netting on the market to consider: polypropylene and high density polyethylene.
Notes on Traps Only using pheromone traps in Mākaha. More are found all the time. Army Natural Resources Program has set up breeding site traps, but nothing in the traps yet.
HDOA is currently making guidelines on how to manage CRB and guiding requirements for laws. One suggestion was that they should also put in verbiage on proper disposal of netting materials.
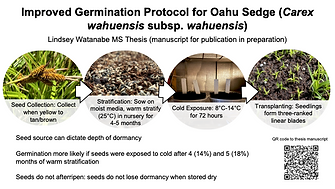
Nursery Germination
Key:
Inoa = Hawaiian name; Germ.= Germination Rate
Tx = Seed treatment
S (Status)
✔️=Working Method: established method, maybe germination rates could be improved upon, but generally satisfactory results
🔄=Trial in Progress
⏳=Future Trial
P (Program)
KR=Kualoa Ranch; NEPM=DOFAW/Native Ecosystems Protection and Management; PPH=Protect and Preserve Hawaiʻi; ANRPO=Army Natural Resources Program, Oʻahu; WV=Waimea Valley, CTAHR=College of Tropical Agriculture and Human Resilience, O. Baldos and L. Watanabe
Do you have similar or varying germination experiences with the species above? Are there other species you are interested in improving your germination rates for? Let us know! This is a working resource and can be expanded in numerous ways.
During the discussion, the group investigated what other information would be useful in a summary table like the one above. An additional category they suggested was potting medium. Is there another category you see as valuable? We want to hear from you on how to keep expanding this to make it a useful resource for native plant growers.
ANRPO Seed Sow Trials
Key:
Inoa = Hawaiian name
Time = Duration of trial
Plots = Number of Plots
Tx = refers to how seeds were treated before being sown
Initial Count = Number of seedlings at first non-zero observation
Final Count = Number of seedlings at last check
SL = Seed Lab
Herbicide Trial Activity
These species have information gaps for in the Weed Control Matrix, and/or were indicated as species of interest through the pre-survey. Each group worked through a worksheet, structured around key steps to setting up an herbicide trial. There were two different types of trials addressed, control method (i.e., what herbicide at what application rate), and control strategy (i.e., we know what herbicide but what are the other management considerations, such as return rate, pairing control with other actions, etc.) Here we provide:
-
the presentation that Oʻahu DOFAW-NEPM gave demonstrating the development and implementation of an herbicide trial to manage Stapelia gigantea at Kāʻena Point, Oʻahu
-
the worksheet used during the activity
-
the list of species that the participants focused on in breakout groups
-
Rubus argutus
-
Spathodea campanulata
-
Ficus spp
-
Trema orientalis
-
Syzygium cumnii
-
Cinamommum burmanii
-
Citharexylum spp
-
Megathyrsus maximums
-
Cenchrus ciliaris
Follow Up
Like what you see? Want to know more? Our intention is that this documentation can help extend the reach of these conversations, or intiate new ones! Let us know what you are thinking, if you have experiences that match or diverge from the ones share here, or if you want to talk to any of these programs/practitioners in more depth.
Do you see an error? A type-o or a misrepresentation of the information you shared? We want to fix it!
You can reach us as EcosystemsExtensionHI@gmail.com or lportner@hawaii.edu and if you haven't already, join the EcosystemsWork listserv (below)!







